A) \[CC{{l}_{4}}\]
B) \[CHC{{l}_{3}}\]
C) \[C{{H}_{2}}C{{l}_{2}}\]
D) \[C{{H}_{3}}Cl\]
Correct Answer: A
Solution :
The molecule in which the bond dipoles of all the bonds are cancel out by each other, is called non-polar eg, \[CC{{l}_{4}}\] In CCl4, there is a large difference between the electronegativities of C and Cl but all the four C - Cl bond dipoles cancel each other, hence it is a non-polar molecule.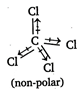
You need to login to perform this action.
You will be redirected in
3 sec
