A) \[C{{O}_{2}}\]
B) \[B{{F}_{3}}\]
C) \[S{{O}_{2}}\]
D) Trans 2-butene
Correct Answer: C
Solution :
Among the given compounds/molecules, only \[\text{S}{{\text{O}}_{\text{2}}}\]has unsymmetrical (angular) structure, thus has the highest dipole moment.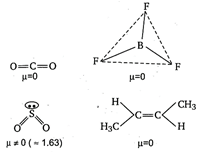
You need to login to perform this action.
You will be redirected in
3 sec
