A) \[Ni{{(CO)}_{4}}\]
B) \[{{[Ni{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
C) \[{{[NiC{{l}_{4}}]}^{2-}}\]
D) \[{{[Ni{{(CN)}_{4}}]}^{2-}}\]
Correct Answer: D
Solution :
The geometry of\[{{[Ni{{(CN)}_{4}}]}^{2-}}\]is square planar. It has\[ds{{p}^{2}}\]hybridisation. It is diamagnetic in nature, i.e., does not have any unpaired electron.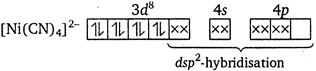
You need to login to perform this action.
You will be redirected in
3 sec
