A) elimination reaction
B) electrophilic addition reaction
C) nucleophilic substitution unimolecular reaction
D) nucleophilic substitution bimolecular reaction
Correct Answer: D
Solution :
The reaction takes place as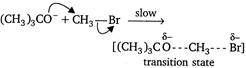 \[\xrightarrow[{}]{fast}\underset{methyl\text{ }tertiary\text{ }butyl\text{ }ether}{\mathop{{{(C{{H}_{3}})}_{3}}C-OC{{H}_{3}}}}\,+B{{r}^{-}}\] Since, in this reaction,\[B{{r}^{-}}\]is substituted by the attack of nucleophile\[{{(C{{H}_{3}})}_{3}}C{{O}^{-}},\]it is a nucleophilic substitution reaction. Moreover, the rate of the reaction depends upon the concentration of\[{{(C{{H}_{3}})}_{3}}C{{O}^{-}}\]and\[C{{H}_{3}}Br\]both, so it is a bimolecular reaction.
\[\xrightarrow[{}]{fast}\underset{methyl\text{ }tertiary\text{ }butyl\text{ }ether}{\mathop{{{(C{{H}_{3}})}_{3}}C-OC{{H}_{3}}}}\,+B{{r}^{-}}\] Since, in this reaction,\[B{{r}^{-}}\]is substituted by the attack of nucleophile\[{{(C{{H}_{3}})}_{3}}C{{O}^{-}},\]it is a nucleophilic substitution reaction. Moreover, the rate of the reaction depends upon the concentration of\[{{(C{{H}_{3}})}_{3}}C{{O}^{-}}\]and\[C{{H}_{3}}Br\]both, so it is a bimolecular reaction.
You need to login to perform this action.
You will be redirected in
3 sec
