A) \[CI{{F}_{3}}\]
B) \[N{{H}_{3}}\]
C) \[B{{F}_{3}}\]
D) \[{{H}_{2}}O\]
Correct Answer: A
Solution :
\[CI{{F}_{3}}\Rightarrow 3bp+2lp,\] ie,\[s{{p}^{3}}d\]hybridisation The shape of\[CI{{F}_{3}}\]should be trigonal bipyramidal but presence of two lone pairs converts it into T-shape.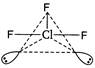 (The shape of\[N{{H}_{3}}\]is pyramidal,\[{{H}_{2}}O\]is angular and that of\[B{{F}_{3}}\]is trigonal planar.)
(The shape of\[N{{H}_{3}}\]is pyramidal,\[{{H}_{2}}O\]is angular and that of\[B{{F}_{3}}\]is trigonal planar.)
You need to login to perform this action.
You will be redirected in
3 sec
