A) outer orbital and diamagnetic
B) inner orbital and paramagnetic
C) inner orbital and diamagnetic
D) outer orbital and paramagnetic
Correct Answer: D
Solution :
In\[{{[Co{{F}_{6}}]}^{4-}},\]central metal Co is present as\[C{{o}^{2+}}\]. \[C{{o}^{2+}}=[Ar]3{{d}^{7}}4{{s}^{0}}\]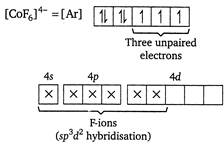 Because of the presence of three unpaired electrons, the complex is paramagnetic. Moreover,\[{{F}^{-}}\]ions occupy outer d-orbitals (ie, nd-orbitals), thus it is an outer orbital complex.
Because of the presence of three unpaired electrons, the complex is paramagnetic. Moreover,\[{{F}^{-}}\]ions occupy outer d-orbitals (ie, nd-orbitals), thus it is an outer orbital complex.
You need to login to perform this action.
You will be redirected in
3 sec
