A) +3, 3d and 4
B) +3, 4d and 6
C) +3, 3d and 6
D) +2, 3d and 6
Correct Answer: C
Solution :
Let the oxidation number of Cr is\[x\]. \[cis[Cr{{(en)}_{2}}C{{l}_{2}}]Cl\] \[x+(0)\times 2+(-1)\times 2-1=0\] \[x-3=0\] \[x=+3\] In\[[Cr{{(en)}_{2}}C{{l}_{2}}]Cl,\]the Cr atom is present as\[C{{r}^{3+}}\]. \[C{{r}^{3+}}=[Ar]\,3{{d}^{3}}4{{s}^{0}}\] \[cis[Cr{{(en)}_{2}}C{{l}_{2}}]=[Ar]\]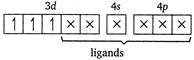 (en being a strong field ligand, can cause pairing.) Thus, 3d orbitals are occupied. Since, two\[Cl\]atoms (monodentate) and two ethylene diammine, ie, en molecules (didentate) are linked two the central atom, the coordination number of central atom
(en being a strong field ligand, can cause pairing.) Thus, 3d orbitals are occupied. Since, two\[Cl\]atoms (monodentate) and two ethylene diammine, ie, en molecules (didentate) are linked two the central atom, the coordination number of central atom You need to login to perform this action.
You will be redirected in
3 sec
