A) tetrahedral
B) square planar
C) trigonal pyramid
D) see-saw
Correct Answer: D
Solution :
In this molecule, S is the central atom with six valence electrons. Out of which four are involved in bonding with four F-atoms and the remaining two behave as a lone pair\[(Ip)\]. The possible arrangement of these pairs are as: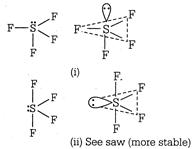 Shape of sulphur tetrafluoride\[(S{{F}_{4}})\]molecule In structure (i) the\[lp\]occupy axial position so there are threelp-bp repulsion at\[90{}^\circ \]. In structure (ii), the\[lp\]occupy an equatorial position and hence there are two\[lp-bp\] repulsions. Hence, arrangement (ii) is more stable. The shape shown in structure (ii) is a distorted tetrahedron, a folded square or a see-saw.
Shape of sulphur tetrafluoride\[(S{{F}_{4}})\]molecule In structure (i) the\[lp\]occupy axial position so there are threelp-bp repulsion at\[90{}^\circ \]. In structure (ii), the\[lp\]occupy an equatorial position and hence there are two\[lp-bp\] repulsions. Hence, arrangement (ii) is more stable. The shape shown in structure (ii) is a distorted tetrahedron, a folded square or a see-saw.
You need to login to perform this action.
You will be redirected in
3 sec
