A) mixture of bromine and HBr
B) \[{{[NiC{{l}_{4}}]}^{2-}}\]
C) bromine
D) none of these
Correct Answer: A
Solution :
\[2KBr+3{{H}_{2}}S{{O}_{4}}+Mn{{O}_{2}}\xrightarrow{\Delta }\] \[2KHS{{O}_{4}}+MnS{{O}_{4}}+2{{H}_{2}}O+B{{r}_{2}}\] \[[N{{i}^{2+}}in\,persence\,of\,CN-=[Ar]\]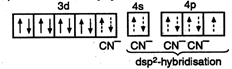 i.e., \[\frac{3-4{{\sin }^{2}}\theta }{1+4{{\sin }^{2}}\theta }=0\] \[\Rightarrow \] \[3-4{{\sin }^{2}}\theta =0\] \[\Rightarrow \] \[\sin \theta =\pm \frac{\sqrt{3}}{2}\] \[=\sin \left( \pm \frac{\pi }{3} \right)\] \[\Rightarrow \] \[\theta =n\pi +{{(-1)}^{n}}\left( \pm \frac{\pi }{3} \right)=n\pi \pm \frac{\pi }{3}\]
i.e., \[\frac{3-4{{\sin }^{2}}\theta }{1+4{{\sin }^{2}}\theta }=0\] \[\Rightarrow \] \[3-4{{\sin }^{2}}\theta =0\] \[\Rightarrow \] \[\sin \theta =\pm \frac{\sqrt{3}}{2}\] \[=\sin \left( \pm \frac{\pi }{3} \right)\] \[\Rightarrow \] \[\theta =n\pi +{{(-1)}^{n}}\left( \pm \frac{\pi }{3} \right)=n\pi \pm \frac{\pi }{3}\]
You need to login to perform this action.
You will be redirected in
3 sec
