A)
![]()
B)
![]()
C)
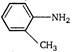
D)
![]()
Correct Answer: D
Solution :
\[C{{H}_{3}}-\](an electron releasing (+1) group) increases electron density at N-atom hence basic nature is increased. (a) (c)
(c) 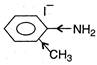 (d)
(d) 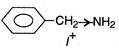 \[{{C}_{6}}{{H}_{5}}\]decreases electron density at N-atom thus basic nature is decreased. (Lone-pair on N in aniline compounds is delocalised along with \[\pi -\]electrons in benzene). Thus, (d) is the strongest base.
\[{{C}_{6}}{{H}_{5}}\]decreases electron density at N-atom thus basic nature is decreased. (Lone-pair on N in aniline compounds is delocalised along with \[\pi -\]electrons in benzene). Thus, (d) is the strongest base.
You need to login to perform this action.
You will be redirected in
3 sec
