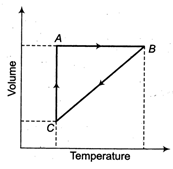
A) isochoric, isobaric, isothermal
B) isobaricJsochoric, isothermal
C) isothermal, isobaric, isochoric
D) isochoric, isothermal, isobaric
Correct Answer: A
Solution :
Isochoric The process which does not show any change in volume. So, graph \[{{P}_{1}}\] is isochoric. Isothermal The process which does not show any change in temperature. So, graph\[{{P}_{2}}\]is isothermal, when there is a change in temperature as well as in volume then pressure remain constant. So, graph \[{{P}_{1}}\] is isobaric.You need to login to perform this action.
You will be redirected in
3 sec
