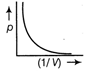
A)
B)
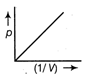
. 
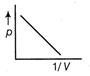
C)
D)
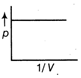
Correct Answer: B
Solution :
From gas equation. \[5\Omega \] where, p is pressure, V is volume, R is gas constant and T is temperature \[\frac{\sqrt{3}}{1}\] ...(i) Equation of straight line is \[\frac{(\sqrt{3}+1)}{(\sqrt{3}-1)}\] ...(ii) where, m is slope and c is constant. Comparing Eqs. (i) and (ii), we get \[\frac{(\sqrt{3}+1)}{1}\] Hence, it is a straight line with positive slope, which passes through origin.You need to login to perform this action.
You will be redirected in
3 sec
