A) \[\text{MgO}\]
B) \[\text{CaO}\]
C) \[C{{H}_{3}}\,\,\overset{+}{\mathop{{{H}_{2}}}}\,\]
D) \[\text{C}{{\text{H}}_{\text{2}}}=\text{CH}\]
Correct Answer: A
Solution :
\[{{k}_{p}}={{k}_{c}}{{(RT)}^{\Delta n}},{{k}_{p}}={{k}_{c}}{{(RT)}^{-1}}\]molecule being symmetrical has zero dipole moment. Replacement of one of the H-atoms by , \[{{k}_{p}}=\frac{{{k}_{c}}}{RT}=\frac{26}{0.082\times 523}=0.6\]-atoms increases the dipolez moment. The increases is rather more than expected because bond moments of \[\text{Bond order }=\frac{\text{Number of bonds}}{\text{Number of resonating structures}}\]bond and \[=\frac{4}{3}=1.33\]bond rain force each other. In \[CaOC{{l}_{2}}+{{H}_{2}}O\xrightarrow{{}}Ca{{(OH)}_{2}}+C{{l}_{2}}\], the resultant of two\[C{{l}_{2}}+2KI\xrightarrow{{}}2KCl+{{I}_{2}}\]bond moments adds to the resultant of C?H and \[{{I}_{2}}+2N{{a}_{2}}{{S}_{2}}{{O}_{3}}\xrightarrow{{}}2NaI\]bond moments.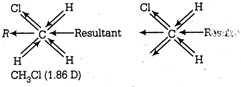
You need to login to perform this action.
You will be redirected in
3 sec
