A) 0.2
B) 0.4
C) 0.6
D) 1.0
Correct Answer: B
Solution :
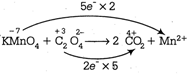 \[2N{{a}_{2}}{{S}_{2}}{{O}_{3}}+{{I}_{2}}\xrightarrow{{}}N{{a}_{2}}{{S}_{4}}{{O}_{6}}+2NaI(D)\]\[n{{s}^{2}}n{{p}^{5}}\] \[(n{{s}^{2}}n{{p}^{6}})\]5(126 g) oxalic acid required = 2 moles\[B{{e}^{2+}}\] 126 g oxalic acid required \[\text{1}{{\text{s}}^{\text{2}}}\] moles = 0.4 moles \[\text{Xe}{{\text{F}}_{\text{2}}},\text{ Xe}{{\text{F}}_{\text{4}}},\text{ XeFg},\text{ Xe}{{\text{O}}_{\text{3}}}\]
\[2N{{a}_{2}}{{S}_{2}}{{O}_{3}}+{{I}_{2}}\xrightarrow{{}}N{{a}_{2}}{{S}_{4}}{{O}_{6}}+2NaI(D)\]\[n{{s}^{2}}n{{p}^{5}}\] \[(n{{s}^{2}}n{{p}^{6}})\]5(126 g) oxalic acid required = 2 moles\[B{{e}^{2+}}\] 126 g oxalic acid required \[\text{1}{{\text{s}}^{\text{2}}}\] moles = 0.4 moles \[\text{Xe}{{\text{F}}_{\text{2}}},\text{ Xe}{{\text{F}}_{\text{4}}},\text{ XeFg},\text{ Xe}{{\text{O}}_{\text{3}}}\]
You need to login to perform this action.
You will be redirected in
3 sec
