A) mixture of secondary and tertiary alcohols
B) mixture of primary and secondary alcohols
C) secondary or tertiary alcohol
D) primary alcohol
Correct Answer: C
Solution :
\[C{{H}_{2}}=C{{H}_{2}}\xrightarrow{{{H}_{2}}O/{{H}^{+}}}\underset{{{1}^{o}}alcohol}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,\] \[C{{H}_{3}}-CH=C{{H}_{2}}\xrightarrow{{{H}_{2}}O/{{H}^{+}}}C{{H}_{3}}\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,HC{{H}_{3}}\] \[{{2}^{o}}\]alcohol through\[{{2}^{o}}\]carbocation\[(C{{H}_{3}}\overset{\oplus }{\mathop{C}}\,HC{{H}_{3}})\] \[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,=C{{H}_{2}}\xrightarrow{{{H}_{2}}O/{{H}^{+}}}{{(C{{H}_{3}})}_{3}}COH\] \[{{3}^{o}}\]alcohol through\[{{3}^{o}}\]carbocation\[[{{(C{{H}_{3}})}_{3}}\overset{\oplus }{\mathop{C}}\,]\] \[C{{H}_{3}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}\,H-CH=C{{H}_{2}}\xrightarrow{{{H}_{2}}O/{{H}^{+}}}\] \[C{{H}_{3}}\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}\,H-CH-C{{H}_{3}}\xrightarrow{rearrangement}C{{H}_{3}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{2}}C{{H}_{3}}\]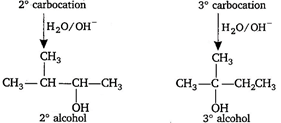
You need to login to perform this action.
You will be redirected in
3 sec
