A) (i) > (ii) > (iii) > (iv)
B) (ii) > (iv) > (iii) > (i)
C) (ii) > (iv) > (i) > (iii)
D) (ii) > (iii) > (iv) > (i)
Correct Answer: D
Solution :
(i)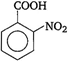 (iii)
(iii)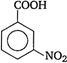 \[-N{{O}_{2}}\]group at any position shows electron withdrawing effect, thus acid strength is increased. But \[o-\]nitro benzoate ion is stabilised by intra molecular \[H-\]bonding like forces, hence its acid strength is maximum. Thus, the order of acid strength is (ii) > (iii) > (iv) > (i).
\[-N{{O}_{2}}\]group at any position shows electron withdrawing effect, thus acid strength is increased. But \[o-\]nitro benzoate ion is stabilised by intra molecular \[H-\]bonding like forces, hence its acid strength is maximum. Thus, the order of acid strength is (ii) > (iii) > (iv) > (i).
You need to login to perform this action.
You will be redirected in
3 sec
