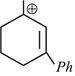
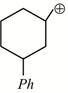
-
question_answer1)
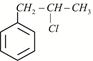
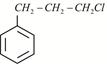
Given: 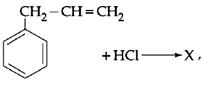 X is:
JEE Main Online Paper (Held On 09 April 2013)
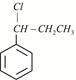
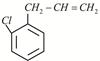
X is:
JEE Main Online Paper (Held On 09 April 2013)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer2) Given: \[\operatorname{X}\operatorname{N}{{\operatorname{a}}_{2}}{{\operatorname{HAsO}}_{3}}+\operatorname{Y}{{\operatorname{NaBrO}}_{3}}+\operatorname{ZHCI}\to \operatorname{NaBr}\]\[\text{+}{{\text{H}}_{\text{2}}}\text{As}{{\text{O}}_{\text{4}}}\text{+NaCl}\] The values of X, Y and Z in above reaction are respectively:
JEE Main Online Paper (Held On 09 April 2013)
A)
2, 1, 2
done
clear
B)
2, 1, 3
done
clear
C)
3, 1, 6
done
clear
D)
3, 1, 4
done
clear
View Answer play_arrow
-
question_answer3) The migration of dispersion medium under the influence of an electric potential is called:
JEE Main Online Paper (Held On 09 April 2013)
A)
Catachresis
done
clear
B)
Electro osmosis
done
clear
C)
Electrophoresis
done
clear
D)
Sedimentation
done
clear
View Answer play_arrow
-
question_answer4) The addition of HI in the presence of peroxide catalyst does not follow anti-Markovnikov?s rule because:
JEE Main Online Paper (Held On 09 April 2013)
A)
HI is a strong reducing agent
done
clear
B)
H-I bond is too strong to be broken homiletically
done
clear
C)
I atom combines with H atom to back HI
done
clear
D)
Iodine atom is not reactive enough to add across a double bond
done
clear
View Answer play_arrow
-
question_answer5) In reaction\[\operatorname{A}+2B\rightleftharpoons 2C+\operatorname{D},\] initial concentration of B was 1.5 tunes of \[\left[ \operatorname{A} \right],\]but at equilibrium the concentrations of A and B became equal. The equilibrium constant for the reaction is:
JEE Main Online Paper (Held On 09 April 2013)
A)
8
done
clear
B)
4
done
clear
C)
12
done
clear
D)
6
done
clear
View Answer play_arrow
-
question_answer6) Trigonal bipyramidal geometry is shown by:
JEE Main Online Paper (Held On 09 April 2013)
A)
\[\operatorname{X}e{{\operatorname{OF}}_{2}}\]
done
clear
B)
\[\operatorname{X}e{{\operatorname{O}}_{3}}{{F}_{2}}\]
done
clear
C)
\[\operatorname{F}\operatorname{X}e\operatorname{O}{{\operatorname{SO}}_{2}}\operatorname{F}\]
done
clear
D)
\[{{\left[ \operatorname{X}\operatorname{e}{{\operatorname{F}}_{8}} \right]}^{2-}}\]
done
clear
View Answer play_arrow
-
question_answer7) If a polythene sample contains two monodisperse fractions in the ration 2: 3 with degree of polymerization 100 and 200, respectively, then its weight average molecular weight will be :
JEE Main Online Paper (Held On 09 April 2013)
A)
4900
done
clear
B)
4600
done
clear
C)
4300
done
clear
D)
5200
done
clear
View Answer play_arrow
-
question_answer8) The instantaneous rate of disappearance of\[{{\operatorname{MnO}}_{4}}^{-}\operatorname{ion}\] in the following reaction is \[4.56\times {{10}^{-3}}\operatorname{M}{{\operatorname{s}}^{-1}}\]
\[2{{\operatorname{MnO}}_{4}}^{-}+10{{\operatorname{I}}^{-}}+16{{H }^{+}}\to 2{{\operatorname{Mn}}^{2}}^{+}+\]
\[5\operatorname{I}+8{{H }_{2}}\operatorname{O}\]
The rate of appearance \[{{\operatorname{I}}_{2}}\] is :
JEE Main Online Paper (Held On 09 April 2013)
A)
\[4.56\times {{10}^{-4}}\operatorname{M}{{\operatorname{s}}^{-1}}\]
done
clear
B)
\[1.14\times {{10}^{-2}}\operatorname{M}{{\operatorname{s}}^{-1}}\]
done
clear
C)
\[1.14\times {{10}^{-3}}\operatorname{M}{{\operatorname{s}}^{-1}}\]
done
clear
D)
\[5.7\times {{10}^{-3}}\operatorname{M}{{\operatorname{s}}^{-1}}\]
done
clear
View Answer play_arrow
-
question_answer9) Which one of the following is most stable?
JEE Main Online Paper (Held On 09 April 2013)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer10) In an atom how many orbital (s) will have the quantum numbers: n=3, \[l=2\]and\[{{m}_{l}}=+2?\]
JEE Main Online Paper (Held On 09 April 2013)
A)
5
done
clear
B)
3
done
clear
C)
1
done
clear
D)
7
done
clear
View Answer play_arrow
-
question_answer11) Potassium dichromate when heated with concentrated sulphuric acid and a soluble chloride, gives brown-red vapours of:
JEE Main Online Paper (Held On 09 April 2013)
A)
\[{{\operatorname{CrO}}_{3}}\]
done
clear
B)
\[\operatorname{Cr}{{\operatorname{CI}}_{3}}\]
done
clear
C)
\[\operatorname{Cr}{{\operatorname{O}}_{3}}{{\operatorname{CI}}_{2}}\]
done
clear
D)
\[{{\operatorname{Cr}}_{2}}{{\operatorname{O}}_{3}}\]
done
clear
View Answer play_arrow
-
question_answer12) Rate of dehydration of alcohols follows the order:
JEE Main Online Paper (Held On 09 April 2013)
A)
\[{{2}^{0}}>{{1}^{0}}>{{\operatorname{CH}}_{3}}\operatorname{OH}>{{3}^{0}}\]
done
clear
B)
\[{{3}^{0}}>{{2}^{0}}>{{1}^{0}}{{\operatorname{CH}}_{3}}\operatorname{OH}\]
done
clear
C)
\[{{2}^{0}}>{{3}^{0}}>{{1}^{0}}{{\operatorname{CH}}_{3}}\operatorname{OH}\]
done
clear
D)
\[{{\operatorname{CH}}_{3}}\operatorname{OH}>{{1}^{0}}>{{2}^{0}}>{{3}^{0}}\]
done
clear
View Answer play_arrow
-
question_answer13) Given (A) \[{{\operatorname{H}}_{2}}(g)+\frac{1}{2}{{O}_{2}}(g)\to {{\operatorname{H}}_{2}}O(l):\] \[\Delta {{\operatorname{H}}^{0}}_{298K}=-285.9\operatorname{k}\operatorname{J}\operatorname{m}\operatorname{o}{{1}^{-1}}\] (B)\[{{H}_{2}}(g)+\frac{1}{2}{{O}_{2}}(g)\to {{H}_{2}}O(g);\] \[\Delta {{H}^{0}}_{298K}=-241.8\operatorname{k}\operatorname{j}\operatorname{mo}{{1}^{-1}}\] The molar enthalpy of vaporization of water will be:
JEE Main Online Paper (Held On 09 April 2013)
A)
\[241.8\,\,kJ\,\,mo{{l}^{-1}}\]
done
clear
B)
\[22.0\,\,kJ\,\,mo{{l}^{-1}}\]
done
clear
C)
\[44.1\,\,kJ\,\,mo{{l}^{-1}}\]
done
clear
D)
\[527.7\,\,kJ\,\,mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
-
question_answer14) Calcination is the process in which:
JEE Main Online Paper (Held On 09 April 2013)
A)
ore is heated above its melting point to expel \[{{H}_{2}}O\] or \[C{{O}_{2}}\] or \[S{{O}_{2}}\]
done
clear
B)
ore is heated below its melting point to expel volatile impurities
done
clear
C)
ore is heated above its melting point to removes S, As and Sb as \[S{{O}_{2}},A{{s}_{2}}{{O}_{3}}\] and\[S{{b}_{2}}{{O}_{3}}\] respectively
done
clear
D)
ore is heated below its melting point to expel \[{{H}_{2}}O\]or\[C{{O}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer15) Sodium Carbonate cannot be used in place of \[{{({{\operatorname{NH}}_{4}})}_{2}}{{\operatorname{CO}}_{3}}\] for the identification of \[C{{a}^{2+}},B{{a}^{2+}}\] and \[\operatorname{S}{{r}^{2+}}\] ions (in group V) during mixture analysis because:
JEE Main Online Paper (Held On 09 April 2013)
A)
\[M{{g}^{2+}}\]ions will also be precipitated
done
clear
B)
Concentration of \[CO_{3}^{2-}\] ions is very low
done
clear
C)
Sodium ions will acid radicals
done
clear
D)
\[{{\operatorname{Na}}^{+}}\] ions will interfere with the detection of \[C{{a}^{2+}},\operatorname{B}{{a}^{2+}},{{\operatorname{Sr}}^{2+}}\] ions
done
clear
View Answer play_arrow
-
question_answer16) Which one of the following molecules is polar?
JEE Main Online Paper (Held On 09 April 2013)
A)
\[\operatorname{Xe}{{\operatorname{F}}_{4}}\]
done
clear
B)
\[{{\operatorname{IF}}_{5}}\]
done
clear
C)
\[\operatorname{Sb}{{\operatorname{F}}_{5}}\]
done
clear
D)
\[\operatorname{C}{{\operatorname{F}}_{4}}\]
done
clear
View Answer play_arrow
-
question_answer17) Type of isomerism which exists between \[\left[ \operatorname{Pd}{{\left( {{\operatorname{C}}_{6}}{{\operatorname{H}}_{5}} \right)}_{2}}{{\left( \operatorname{SCN} \right)}_{2}} \right]\]and \[\left[ \operatorname{Pd}{{\left( {{\operatorname{C}}_{6}}{{\operatorname{H}}_{5}} \right)}_{2}}{{\left( \operatorname{NCS} \right)}_{2}} \right]\] is
JEE Main Online Paper (Held On 09 April 2013)
A)
Linkage isomerism
done
clear
B)
Coordination isomerism
done
clear
C)
lionization isomerism
done
clear
D)
Solvate isomerism
done
clear
View Answer play_arrow
-
question_answer18) In which of the following ionization processes the bond energy has increased and also the magnetic behaviour has changed from paramagnetic to diamagnetic?
JEE Main Online Paper (Held On 09 April 2013)
A)
\[ N\operatorname{O}\to {{\operatorname{NO}}^{+}}\]
done
clear
B)
\[{{N }_{2}}\to {{\operatorname{N}}_{2}}^{+}\]
done
clear
C)
\[{{C}_{2}}\to {{C}_{2}}^{+}\]
done
clear
D)
\[{{O}_{2}}\to {{O}_{2}}^{+}\]
done
clear
View Answer play_arrow
-
question_answer19) 12g of a nonvolatile solute dissolved in 108g of water produces the relative lowering of vapour pressure of 0.1. The molecular mass of the lute is:
A)
80
done
clear
B)
60
done
clear
C)
20
done
clear
D)
40
done
clear
View Answer play_arrow
-
question_answer20) Which of the following enzyme converts starch into maltose?
JEE Main Online Paper (Held On 09 April 2013)
A)
Diastase
done
clear
B)
Maltase
done
clear
C)
Zymase
done
clear
D)
Invertase
done
clear
View Answer play_arrow
-
question_answer21) Electrode potentials \[\left( {{E}^{0}} \right)\] are given below: \[{{\operatorname{Cu}}^{+}}+\operatorname{Cu}=+0.52V,\] \[F{{e}^{3+}}/F{{e}^{2+}}=+0.77V,\] \[\frac{1}{2}{{\operatorname{I}}_{2}}\left( s \right)/{{I}^{-}}=+0.54V,\] \[A{{g}^{+}}/Ag=+0.88V.\] Based on the above potentials, strongest oxidizing agent will be:
JEE Main Online Paper (Held On 09 April 2013)
A)
\[{{\operatorname{Cu}}^{+}}\]
done
clear
B)
\[{{\operatorname{Fe}}^{3}}^{+}\]
done
clear
C)
\[{{\operatorname{Ag}}^{+}}\]
done
clear
D)
\[{{\operatorname{I}}_{2}}\]
done
clear
View Answer play_arrow
-
question_answer22) Aryl fluoride may be prepared from arene diazonium chloride using:
JEE Main Online Paper (Held On 09 April 2013)
A)
\[{{\operatorname{HBF}}_{4}}/\Delta \]
done
clear
B)
\[{{\operatorname{HBF}}_{4}}/\operatorname{N}a\operatorname{N}{{\operatorname{O}}_{2}},\operatorname{Cu},\Delta \]
done
clear
C)
\[\operatorname{Cu}\operatorname{F}/\operatorname{H}\operatorname{F}\]
done
clear
D)
\[\operatorname{Cu}/\operatorname{H}\operatorname{F}\]
done
clear
View Answer play_arrow
-
question_answer23) Anether (A) \[{{\operatorname{C}}_{5}}{{\operatorname{H}}_{12}}\operatorname{O},\]when heated with excess of hot concentrated HI produced two alky halides which when treated with NaOH yielded compounds (B) and (C) Oxidation of (B) and (C) gave a propanone and an ethanoic acid respectively. The IUPAC name of the ether (A) is:
JEE Main Online Paper (Held On 09 April 2013)
A)
2-ethoxypropane
done
clear
B)
ethoxypropane
done
clear
C)
methoxybutane
done
clear
D)
2-methoxybutane
done
clear
View Answer play_arrow
-
question_answer24) Electron gain enthalpy with negative sing of fluorine is less than that of chlorine due to:
JEE Main Online Paper (Held On 09 April 2013)
A)
High ionization enthalpy of fluorine
done
clear
B)
Smaller size of chlorine atom
done
clear
C)
Smaller size of fluorine atom
done
clear
D)
Bigger size of \[2p\]orbital of fluorine
done
clear
View Answer play_arrow
-
question_answer25) Solid \[Ba{{(N{{O}_{3}})}_{2}}\] is gradually dissolved in a \[1.0\times {{10}^{-4}}\,M\,N{{a}_{2}}C{{O}_{3}}\] solution. At which concentration of \[B{{a}^{2+}}\], precipitate of \[BaC{{O}_{3}}\]begins to form? \[({{K}_{sp}}\] for \[BaC{{O}_{3}}=5.1\times {{10}^{-9}})\]
JEE Main Online Paper (Held On 09 April 2013)
A)
\[5.1\times {{10}^{-5}}\operatorname{M}\]
done
clear
B)
\[7.1\times {{10}^{-8}}\operatorname{M}\]
done
clear
C)
\[4.1\times {{10}^{-5}}\operatorname{M}\]
done
clear
D)
\[8.1\times {{10}^{-7}}\operatorname{M}\]
done
clear
View Answer play_arrow
-
question_answer26) An element having an atomic radius of 0.14 nm crystallizes in a fcc unit cell. What is the length of a side of the cell?
JEE Main Online Paper (Held On 09 April 2013)
A)
0.56 nm
done
clear
B)
0.24 nm
done
clear
C)
0.96 nm
done
clear
D)
0.4 nm
done
clear
View Answer play_arrow
-
question_answer27) Which of the following compounds is not expected to show Lassaignes? test for nitrogen?
JEE Main Online Paper (Held On 09 April 2013)
A)
Propanenitrile
done
clear
B)
Hydroxylamie hydrochloride
done
clear
C)
Niromethane
done
clear
D)
Ethanmine
done
clear
View Answer play_arrow
-
question_answer28) Formaldehyde can be distinguished from acetaldehyde the use of:
JEE Main Online Paper (Held On 09 April 2013)
A)
Schiff's reagent
done
clear
B)
Tollen's reagent
done
clear
C)
\[{{\operatorname{I}}_{2}}/\]Alkali
done
clear
D)
Fehling's solution
done
clear
View Answer play_arrow
-
question_answer29) By how many folds the temperature of a gas would increase when the root mean square velocity of the gas molecules in a container of fixed volume is increased from \[5\times {{10}^{4}}\operatorname{cm}/s\] to\[10\times {{10}^{4}}\] cm/s?
JEE Main Online Paper (Held On 09 April 2013)
A)
Two
done
clear
B)
Three
done
clear
C)
Six
done
clear
D)
Four
done
clear
View Answer play_arrow
-
question_answer30) The element which of the following outer electron configuration may exhibit the largest number of oxidation states in its compounds:
JEE Main Online Paper (Held On 09 April 2013)
A)
\[3{{\operatorname{d}}^{5}}4{{\operatorname{s}}^{2}}\]
done
clear
B)
\[3{{\operatorname{d}}^{8}}4{{\operatorname{s}}^{2}}\]
done
clear
C)
\[3{{\operatorname{d}}^{7}}4{{\operatorname{s}}^{2}}\]
done
clear
D)
\[3{{\operatorname{d}}^{6}}4{{\operatorname{s}}^{2}}\]
done
clear
View Answer play_arrow
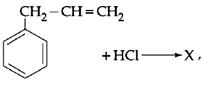 X is:
JEE Main Online Paper (Held On 09 April 2013)
X is:
JEE Main Online Paper (Held On 09 April 2013)