-
question_answer1) The conversion of benzene diazonium chloride to bromobenzene can be accomplished by
JEE Main Online Paper (Held On 12 May 2012)
A)
Reimer- Tiemann reacton
done
clear
B)
Friedel-Crafts reaction
done
clear
C)
Gattermann reaction
done
clear
D)
Azo-eoupling reaction
done
clear
View Answer play_arrow
-
question_answer2) The radius of a calcium ion is 94 pm and of the oxide ion is 146 pm. The possible crystal structure of calcium oxide will be
JEE Main Online Paper (Held On 12 May 2012)
A)
tetrahedral
done
clear
B)
trigonal
done
clear
C)
octahedral
done
clear
D)
pyramidal
done
clear
View Answer play_arrow
-
question_answer3) The formation of molecular complex \[B{{F}_{3}}-N{{H}_{3}}\]results in a change in hybridization of boron
JEE Main Online Paper (Held On 12 May 2012)
A)
from sp2 to dsp2
done
clear
B)
from sp2 to sp3
done
clear
C)
from sp3 to sp2
done
clear
D)
from sp3 to sp3
done
clear
View Answer play_arrow
-
question_answer4) A metal Mon heating in nitrogen gas gives Y. Y on treatment with \[{{H}_{2}}O\] gives a colourless gas which when passed through \[CuS{{O}_{4}}\] solution gives a blue colour. V is
JEE Main Online Paper (Held On 12 May 2012)
A)
\[N{{H}_{3}}\]
done
clear
B)
\[Mg{{(N{{O}_{3}})}_{2}}\]
done
clear
C)
\[M{{g}_{3}}{{N}_{2}}\]
done
clear
D)
\[MgO\]
done
clear
View Answer play_arrow
-
question_answer5) Water sample is reported to be highly polluted if BOD (Biological Oxygen Demand) value of sample becomes
JEE Main Online Paper (Held On 12 May 2012)
A)
more than 17 ppm
done
clear
B)
equal to 10 ppm
done
clear
C)
equal to 5 ppm
done
clear
D)
less than 5 ppm
done
clear
View Answer play_arrow
-
question_answer6) A solution containing 0.85 g of \[znC{{l}_{2}}\] in 125.0 g of water freezes at \[-0.23{}^\circ C\]. The apparent degree of dissociation of the salt is (\[{{K}_{f}}\] for water = 1.86 K kg \[\text{mo}{{\text{l}}^{-1}}\], atomic mass: Zn = 65.3 and Cl = 35.5)
JEE Main Online Paper (Held On 12 May 2012)
A)
1.36%
done
clear
B)
73.5%
done
clear
C)
7.35%
done
clear
D)
2.47%
done
clear
View Answer play_arrow
-
question_answer7) Which of the following complex ions will exhibit optical isomerism? (en = 1,2-diamine ethane).
JEE Main Online Paper (Held On 12 May 2012)
A)
\[{{[Cr{{(N{{H}_{3}})}_{2}}C{{l}_{2}}]}^{+}}\]
done
clear
B)
\[{{[Co{{(en)}_{2}}C{{l}_{2}}]}^{+}}\]
done
clear
C)
\[{{[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]}^{+}}\]
done
clear
D)
\[{{[Zn{{(en)}_{2}}]}^{2+}}\]
done
clear
View Answer play_arrow
-
question_answer8) Which among the following elements has the highest first ionization enthalpy?
JEE Main Online Paper (Held On 12 May 2012)
A)
Nitrogen
done
clear
B)
Boron
done
clear
C)
Carbon
done
clear
D)
Oxygen
done
clear
View Answer play_arrow
-
question_answer9) Magnetic moment of \[G{{d}^{3+}}ion(Z=64)\]is
JEE Main Online Paper (Held On 12 May 2012)
A)
3.62 BM
done
clear
B)
9.72 BM
done
clear
C)
7.9 BM
done
clear
D)
10.60 BM
done
clear
View Answer play_arrow
-
question_answer10) An aqueous solution of oxalic acid dehydrate contains its 6.3 g in 250 ml. The volume of 0.1N NaOH required to completely neutralize 10 ml of this solution
JEE Main Online Paper (Held On 12 May 2012)
A)
4ml
done
clear
B)
20ml
done
clear
C)
2ml
done
clear
D)
40ml
done
clear
View Answer play_arrow
-
question_answer11) In the electrolysis of alumina to obtain aluminium metal, cryolite is added mainly to
JEE Main Online Paper (Held On 12 May 2012)
A)
lower the melting point of alumina
done
clear
B)
dissolve alumina in molten cryolite
done
clear
C)
remove the impurities of alumina
done
clear
D)
increase the electrical conductivity
done
clear
View Answer play_arrow
-
question_answer12) Synthetic polymer bakelite can be prepared from following compounds
JEE Main Online Paper (Held On 12 May 2012)
A)
Styrene and vinyl chloride
done
clear
B)
Acrylonitrile and vinyl chloride
done
clear
C)
Adipic acid and ethylene glycol
done
clear
D)
Phenol and formaldehyde
done
clear
View Answer play_arrow
-
question_answer13) The correct statement for both the processes of physisorption and chemisorption is
JEE Main Online Paper (Held On 12 May 2012)
A)
both are endothermic
done
clear
B)
chemisorption is endothermic but physisorption is exothermic
done
clear
C)
both are exothermic
done
clear
D)
physisorption is endothermic but chemisorption is exothermic.
done
clear
View Answer play_arrow
-
question_answer14) Which of the following statements is wrong?
JEE Main Online Paper (Held On 12 May 2012)
A)
Ethyl chloride on reduction with Zn-Cu couple and alcohol gives ethane.
done
clear
B)
The reaction of methyl magnesium bromide with acetone gives butano1-2.
done
clear
C)
Alkyl halides follow the following reactivity sequence on reaction with alkeries. \[R-I>R-Br>R-Cl>R-I\]
done
clear
D)
\[{{C}_{2}}{{H}_{4}}C{{l}_{2}}\] may exist in two isomeric forms
done
clear
View Answer play_arrow
-
question_answer15) Although \[C{{N}^{-}}\]ion and \[{{N}_{2}}\] molecule are isoelectronic, yet \[{{N}_{2}}\] molecule is chemically inert because of
JEE Main Online Paper (Held On 12 May 2012)
A)
presence of more number of electrons in bonding orbitals
done
clear
B)
lone bond energy
done
clear
C)
absence of bond polarity
done
clear
D)
uneven electron distribution.
done
clear
View Answer play_arrow
-
question_answer16) Amylopectin is a polymer of
JEE Main Online Paper (Held On 12 May 2012)
A)
\[\alpha -D-\] glucose
done
clear
B)
amino acid
done
clear
C)
\[\beta -D-\] glucose
done
clear
D)
amylase.
done
clear
View Answer play_arrow
-
question_answer17) If the radius of first orbit of H atom is \[{{a}_{0}},\] the de- Broglie wavelength of an electron in the third orbit is
JEE Main Online Paper (Held On 12 May 2012)
A)
\[4\pi {{a}_{0}}\]
done
clear
B)
\[8\pi {{a}_{0}}\]
done
clear
C)
\[6\pi {{a}_{0}}\]
done
clear
D)
\[2\pi {{a}_{0}}\]
done
clear
View Answer play_arrow
-
question_answer18) Chemically heroin is
JEE Main Online Paper (Held On 12 May 2012)
A)
morphine monoacetate
done
clear
B)
morphine dibenzbate
done
clear
C)
morphine diacetate
done
clear
D)
morphine monobenzoate
done
clear
View Answer play_arrow
-
question_answer19)
In the given reaction, 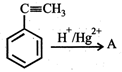 the product 'A' is
JEE Main Online Paper (Held On 12 May 2012)
the product 'A' is
JEE Main Online Paper (Held On 12 May 2012)
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer20) The ppm level of \[{{F}^{-}}\]in a 500 g sample of a tooth paste containing 0.2 g \[{{F}^{-}}\]is
JEE Main Online Paper (Held On 12 May 2012)
A)
400
done
clear
B)
1000
done
clear
C)
250
done
clear
D)
200
done
clear
View Answer play_arrow
-
question_answer21) In a chemical reaction A is converted into B. The rates of reaction, starting with initial concentrations of A as \[2\times {{10}^{-3}}M\] and \[1\times {{10}^{-3}}M,\]are equal to \[2.40\times {{10}^{-4}}M{{s}^{-1}}\]and \[0.60\times {{10}^{-4}}M{{s}^{-1}}\] respectively. The order of reaction with respect to reactant A will be
JEE Main Online Paper (Held On 12 May 2012)
A)
0
done
clear
B)
1.5
done
clear
C)
1
done
clear
D)
2
done
clear
View Answer play_arrow
-
question_answer22) 8 mol of\[A{{B}_{3}}(g)\]are introduced into a 1.0 dm3 vessel. If it dissociates as \[2A{{B}_{3}}(g){{A}_{2}}(g)+3{{B}_{2}}(g).\]At equilibrium, 2 mol of \[{{A}_{2}}\] are found to be present. The equilibrium constant of this reaction is
JEE Main Online Paper (Held On 12 May 2012)
A)
2
done
clear
B)
3
done
clear
C)
27
done
clear
D)
36
done
clear
View Answer play_arrow
-
question_answer23) 5 g of benzene on nitration gave 6.6 g of nitrobenzene. The theoretical yield of the nitrobenzene will be
JEE Main Online Paper (Held On 12 May 2012)
A)
4.5 g
done
clear
B)
5.6g
done
clear
C)
8.09g
done
clear
D)
6.6g
done
clear
View Answer play_arrow
-
question_answer24) Given (i)\[HCN(aq)+{{H}_{2}}O(l){{H}_{3}}{{O}^{+}}(aq)+C{{N}^{-}}(aq)\]\[{{K}_{a}}=6.2\times {{10}^{-10}}\] (ii)\[C{{N}^{-}}(aq)+{{H}_{2}}O(l)HCN(aq)+O{{H}^{-}}(aq)\] \[{{K}_{b}}=1.6\times {{10}^{-5}}.\] These equilibria show the following order of the relative base strength,
JEE Main Online Paper (Held On 12 May 2012)
A)
\[OH>{{H}_{2}}O>C{{N}^{-}}\]
done
clear
B)
\[O{{H}^{-}}>C{{N}^{-}}>{{H}_{2}}O\]
done
clear
C)
\[{{H}_{2}}O>C{{N}^{-}}>O{{H}^{-}}\]
done
clear
D)
\[C{{N}^{-}}>{{H}_{2}}O>O{{H}^{-}}\]
done
clear
View Answer play_arrow
-
A)
(iv)>(i)>(ii)>(iii)
done
clear
B)
(ii)>(iii)>(i)>(iv)
done
clear
C)
(iii)>(i)>(iv)>(ii)
done
clear
D)
(i)>(ii)>(iii)>(iv)
done
clear
View Answer play_arrow
-
question_answer26) The difference between the reaction enthalpy change \[({{\Delta }_{r}}H)\]arid reaction internal energy change \[({{\Delta }_{r}}U)\] for the reaction: \[2{{C}_{6}}{{H}_{6}}(l)+15{{O}_{2}}(g)\xrightarrow[{}]{{}}12C{{O}_{2}}(g)+6{{H}_{2}}O(l)\]at 300 K is \[(R=8.314\,J\,mo{{l}^{-1}}\,{{K}^{-1}})\]
JEE Main Online Paper (Held On 12 May 2012)
A)
\[0\,J\,mo{{l}^{-1}}\,\]
done
clear
B)
\[2490\,J\,mo{{l}^{-1}}\,\]
done
clear
C)
\[-2490\,J\,mo{{l}^{-1}}\,\]
done
clear
D)
\[-7482\,J\,mo{{l}^{-1}}\,\]
done
clear
View Answer play_arrow
-
question_answer27) \[C{{H}_{3}}CHO\xrightarrow[Zn\left( Hg \right)/Conc.HCl]{[H]}C{{H}_{3}}C{{H}_{3}}\]The reaction;
JEE Main Online Paper (Held On 12 May 2012)
A)
Cannizaro's reaction
done
clear
B)
Rosenmund reduction
done
clear
C)
Wolf-Kishner reduction
done
clear
D)
Clemmenson reduction
done
clear
View Answer play_arrow
-
question_answer28) \[\alpha ,v\]and u represent most probable velocity, average velocity and root mean square velocity respectively of a gas at a particular temperature. The correct order among the following is
JEE Main Online Paper (Held On 12 May 2012)
A)
\[u>v>\alpha \]
done
clear
B)
\[v>u>\alpha \]
done
clear
C)
\[\alpha >u>v\]
done
clear
D)
\[u>\alpha >v\]
done
clear
View Answer play_arrow
-
question_answer29) Among the following chloro-compound having the lowest dipole moment is
JEE Main Online Paper (Held On 12 May 2012)
A)
\[C{{H}_{3}}Cl\]
done
clear
B)
done
clear
C)
\[C{{H}_{2}}C{{l}_{2}}\]
done
clear
D)
done
clear
View Answer play_arrow
-
question_answer30) In the following balanced reaction,\[X\,MnO_{4}^{-}+Y\,{{C}_{2}}O_{4}^{2-}+Z{{H}^{+}}\]\[X\,M{{n}^{2+}}+2Y\,C{{O}_{2}}+\frac{Z}{2}{{H}_{2}}O\]values of X, Y and Z respectively are
JEE Main Online Paper (Held On 12 May 2012)
A)
2,5,16
done
clear
B)
8,2,5
done
clear
C)
5,2,16
done
clear
D)
5,8,4
done
clear
View Answer play_arrow
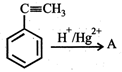 the product 'A' is
JEE Main Online Paper (Held On 12 May 2012)
the product 'A' is
JEE Main Online Paper (Held On 12 May 2012)
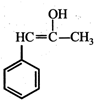
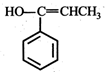
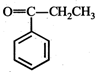
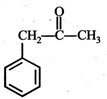
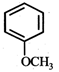
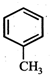 (iii) (iv)
(iii) (iv) 