A)
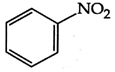
B)
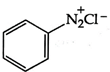
C)
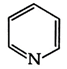
D)
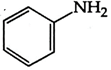
Correct Answer: D
Solution :
Diazonium, nitro and ring containing nitrogen do not show Kjeldahl method because it is not interconvert into ammonium ion so the answer will be (3) \[\text{Ph-N}{{\text{H}}_{\text{2}}}\xrightarrow{{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}}{{\text{(N}{{\text{H}}_{\text{4}}}\text{)}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\xrightarrow{{}}\text{Kjeldahl method}\]You need to login to perform this action.
You will be redirected in
3 sec
