A) \[Cl{{F}_{3}}\]and \[IO_{4}^{-}\]
B) \[lCl_{2}^{-}\] and \[lC{{l}_{5}}\]
C) \[lO_{3}^{-}\] and \[l{{O}_{2}}F_{2}^{-}\]
D) \[XeO{{F}_{2}}\]and \[XeO{{F}_{4}}\]
Correct Answer: D
Solution :
According, to VSEPR theory \[Xe\](in \[XeO{{F}_{2}}\]) Has fine electron pairs with three bonding pair. This means its geometry is T-Shaped. Similarly, \[Xe\]in \[XeO{{F}_{4}}\] has a hybridizah on of \[s{{p}^{3}}{{d}^{2}}\] Thus its geometry is square-pyramidal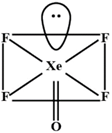
You need to login to perform this action.
You will be redirected in
3 sec
