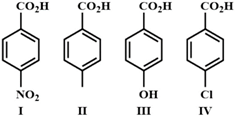
A) \[III<II<IV<I\]
B) \[I<III<II<IV\]
C) \[IV<II<III<I\]
D) \[II<IV<III<I\]
Correct Answer: A
Solution :
The increasing order of the acidity of the carboxylic acids is \[III<II<IV<I\]. In aromatic acids, electron withdrawing groups like \[-Cl,-CN,-N{{O}_{2}}\] increases the acidity whereas electron releasing groups like \[-C{{H}_{3}},-OH,-OC{{H}_{3}},-N{{H}_{2}}\] decreases the acidity.You need to login to perform this action.
You will be redirected in
3 sec
