A) 5.91 BM
B) 3.87 BM
C) 1.73 BM
D) 2.82 BM
Correct Answer: D
Solution :
In\[{{[Cr(NO)(N{{H}_{3}}){{(CN)}_{4}}]}^{2-}},\]\[C{{r}^{2+}}({{d}^{4}})\]is given as :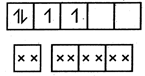 i.e., 2 unpaired electrons \[\mu =\sqrt{2(2+2)}=\sqrt{8}=2.82\]
i.e., 2 unpaired electrons \[\mu =\sqrt{2(2+2)}=\sqrt{8}=2.82\]
You need to login to perform this action.
You will be redirected in
3 sec
