A) \[{{10}^{8}}N/{{m}^{2}}\]
B) \[{{10}^{4}}N/{{m}^{2}}\]
C) \[{{10}^{3}}N/{{m}^{2}}\]
D) \[{{10}^{16}}N/{{m}^{2}}\]
Correct Answer: C
Solution :
Note : Pressure is defined as normal force per unit area. Force is calculated as change in momentum/ time By this answer is \[2N/{{m}^{2}}\] None of the option matches so this question must be Bonus Detailed solution is as following.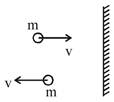 Magnitude of change in momentum per collision = 2mv Pressure \[=\frac{Force}{Area}=\frac{N(2mv)}{1}\] \[=\frac{{{10}^{22}}\times 2\times {{10}^{-26}}\times {{10}^{4}}}{1}\] \[=2N/{{m}^{2}}\]
Magnitude of change in momentum per collision = 2mv Pressure \[=\frac{Force}{Area}=\frac{N(2mv)}{1}\] \[=\frac{{{10}^{22}}\times 2\times {{10}^{-26}}\times {{10}^{4}}}{1}\] \[=2N/{{m}^{2}}\]
You need to login to perform this action.
You will be redirected in
3 sec
