A) \[CHB{{r}_{3}}~\]
B) \[CHC{{l}_{3}}\]
C) \[CH{{\left( CN \right)}_{3}}\]
D) \[CH{{I}_{3}}\]
Correct Answer: C
Solution :
Acidic strength \[\propto \] stability of Anion after loosing proton \[\propto \] EWG - I, - M, -H (1) \[CHB{{r}_{3}}\,\,\to \,\,\overset{\Theta }{\mathop{C}}\,B{{r}_{3}}\] (2) \[CHC{{l}_{3}}\,\,\to \,\,\overset{\Theta }{\mathop{C}}\,C{{l}_{3}}\] (3)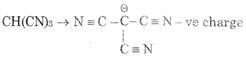 stabilize by - M of three C \[\equiv \] N group (4) \[CH{{I}_{3}}\text{ }\to \text{ }\overset{\Theta }{\mathop{C}}\,{{I}_{3}}\] 3 is most acidic
stabilize by - M of three C \[\equiv \] N group (4) \[CH{{I}_{3}}\text{ }\to \text{ }\overset{\Theta }{\mathop{C}}\,{{I}_{3}}\] 3 is most acidic
You need to login to perform this action.
You will be redirected in
3 sec
