A) potassium amminetrichloroplatinate (II)
B) potassium tris(oxalato) chormate (III)
C) aquachlorobis (ethylenediamine) cobalt (II) chloride
D) pentaquachlorochromium (III) chloride
Correct Answer: C
Solution :
Aquachlorobis (ethylenediamine) cobalt (II) chloride will show geometrical (cis-trans) isomerism. In cis isomer, two ethylene diamine ligands are adjacent and in trans isomer, they are opposite to each other.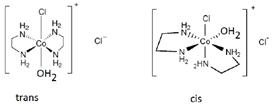 Aquachlorobis (ethylenediamine) cbalt (II) chloride
Aquachlorobis (ethylenediamine) cbalt (II) chloride
You need to login to perform this action.
You will be redirected in
3 sec
