 is
JEE Main Online Paper (Held On 19 May 2012)
is
JEE Main Online Paper (Held On 19 May 2012)
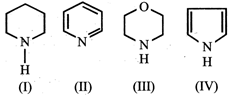
A) \[IV>I>III>II\]
B) \[I>III>II>IV\]
C) \[III>I>IV>II\]
D) \[II>I>III>IV\]
Correct Answer: B
Solution :
The order of basicity is I > III > II > IV The lone pair of electrons on N is more readily available for protonation in I and III then in II. Ill contains an oxygen atom which has - I effect due to which it is less basic than I. In compound IV lone pair of \[{{e}^{-}}s\] on N-atom is contributed towards the aromatic sextet formation and hence is not at all available for protonation. Hence option (b) is correct.You need to login to perform this action.
You will be redirected in
3 sec
