A) \[{{[Co{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
B) \[{{[Cr{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
C) \[{{[Mn{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
D) \[{{[Fe{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
Correct Answer: A
Solution :
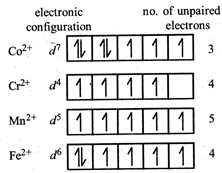 Since \[C{{o}^{3+}}\] has lowest no. of unpaired electrons hence lowest paramagnetic behaviour is shown by \[{{[Co{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
Since \[C{{o}^{3+}}\] has lowest no. of unpaired electrons hence lowest paramagnetic behaviour is shown by \[{{[Co{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
You need to login to perform this action.
You will be redirected in
3 sec
