| \[M(s)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow[{}]{{}}MO(s)\]and |
| \[C(s)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow[{}]{{}}CO(s)\] |
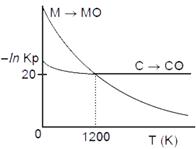 |
A) At T > 1200 K, carbon will reduce MO(s) to M(s).
B) At T < 1200 K, oxidation of carbon is unfavourable.
C) Oxidation of carbon is favourable at all temperatures.
D) At \[T<1200K,\] the reaction \[MO(s)+C(s)\to M(s)+CO(g)\]is spontaneous.
Correct Answer: D
Solution :
According to Ellingham diagram, as given At \[T<1200,\]carbon will reduce MO(s) to M(s) hence, chemical reaction\[{{C}_{(s)}}+M{{O}_{(s)}}\xrightarrow[{}]{{}}{{M}_{(s)}}+C{{O}_{(g)}}\] is spontaneous.
At \[T<1200,\]carbon will reduce MO(s) to M(s) hence, chemical reaction\[{{C}_{(s)}}+M{{O}_{(s)}}\xrightarrow[{}]{{}}{{M}_{(s)}}+C{{O}_{(g)}}\] is spontaneous.
You need to login to perform this action.
You will be redirected in
3 sec
