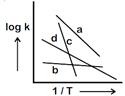
A) \[{{E}_{b}}>{{E}_{a}}>{{E}_{d}}>{{E}_{c}}\]
B) \[{{E}_{c}}>{{E}_{a}}>{{E}_{d}}>{{E}_{b}}\]
C) \[{{E}_{a}}>{{E}_{c}}>{{E}_{d}}>{{E}_{b}}\]
D) \[{{E}_{b}}>{{E}_{d}}>{{E}_{c}}>{{E}_{a}}\]
Correct Answer: B
Solution :
\[logk=logA\frac{{{E}_{a}}}{2.303\,\,RT}\] \[Slope=\frac{{{E}_{a}}}{2.303\,\,R}\] So correct order of activation energies \[\Rightarrow \text{ }{{E}_{c}}>{{E}_{a}}>{{E}_{d}}>{{E}_{b}}\]You need to login to perform this action.
You will be redirected in
3 sec
