 JEE Main Solved Paper-2014
JEE Main Solved Paper-2014
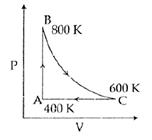
A) The change in internal energy in the process AB is − 350 R.
B) The change in internal energy in the process BC is − 500 R.
C) The change in internal energy in whole cyclic process is 250 R.
D) The change in internal energy in the process CA is 700 R.
Correct Answer: B
Solution :
\[\underline{AB}\]\[\Delta U=n{{C}_{v}}\Delta T=1\cdot \frac{5R}{2}(800-400)=1000R\] Option (1) is not correct \[\underline{CA}\]\[\Delta U=n{{C}_{v}}\Delta T=1\cdot \frac{5R}{2}(400-600)=-500R\] Option (4) is not correct\[\Delta {{U}_{cycle}}=0\] \[\therefore \]\[\Delta {{U}_{AB}}+\Delta {{U}_{BC}}+\Delta {{U}_{CA}}=0\] \[1000R+\Delta {{U}_{AB}}-\Delta {{U}_{BC}}-500R=0\] \[\Delta {{U}_{BC}}=-500R\]You need to login to perform this action.
You will be redirected in
3 sec
