A) 80 K
B) - 73 K
C) 3 K
D) 20 K
Correct Answer: D
Solution :
| [d] The rms velocity of the molecule of a gas of molecular weight M at kelvin temperature T is given by |
| Let |
| i.e., |
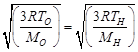 |
| Hence, |
| Given, |
| |
You need to login to perform this action.
You will be redirected in
3 sec
