| One mole of diatomic ideal gas undergoes a cyclic process ABC as shown in figure. The process BC is adiabatic. The temperatures at A, B and C are 400 K, 800 K and 600 K respectively. Choose the correct statement: [JEE MAIN 2014] |
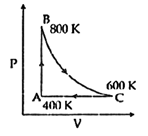 |
A) The change in internal energy in the process AB is − 350 R.
B) The change in internal energy in the process BC is − 500 R.
C) The change in internal energy in whole cyclic process is 250 R.
D) The change in internal energy in the process CA is 700 R.
Correct Answer: B
Solution :
| [b] |
| |
| Option (1) is not correct |
| |
| Option (4) is not correct |
| |
| |
| |
You need to login to perform this action.
You will be redirected in
3 sec
