| A solid body of constant heat capacity 1J/°C is being heated by keeping it in contact with reservoirs in two ways: |
| (i) Sequentially keeping in contact with 2 reservoirs such that each reservoir supplies same amount of heat. |
| (ii) Sequentially keeping in contact with 8 reservoirs such that each reservoir supplies same amount of heat. |
| In both the cases body is brought from initial temperature 100°C to final temperature 200°C. Entropy change of the body in the two cases respectively is: [JEE MAIN 2015] |
A) \[\ln \,2,\,2\ln \,2\]
B) \[2\ln 2,\,\,8\ln \,2\]
C) \[\ln \,2,\,4\ln \,2\]
D) \[\ln \,2,\,\ln \,2\]
Correct Answer: D
Solution :
[d] 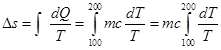 |
| or |
| |
You need to login to perform this action.
You will be redirected in
3 sec
