A) \[sp\]
B) \[s{{p}^{2}}\]
C) \[s{{p}^{3}}\]
D) \[s{{p}^{3}}d\]
Correct Answer: B
Solution :
In ethylene \[(C{{H}_{2}}=C{{H}_{2}}),\] both carbon remains \[s{{p}^{2}}-\]hybridized. C in excited state \[=\overset{2s}{\mathop{}}\,\,\,\,\overset{2p}{\mathop{}}\,\] \[{{C}_{2}}{{H}_{4}}=\underset{{{H}_{1s}}}{\mathop{\underset{|}{\mathop{{{(s{{p}^{2}})}^{1}}}}\,}}\,\underset{{{H}_{1s}}}{\mathop{\underset{|}{\mathop{{{(s{{p}^{2}})}^{1}}}}\,}}\,\underset{{{(s{{p}^{2}})}^{1}}}{\mathop{\underset{|}{\mathop{{{(s{{p}^{2}})}^{1}}}}\,}}\,2p_{z}^{1}\]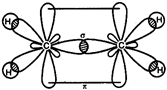
You need to login to perform this action.
You will be redirected in
3 sec
