A) \[{{H}_{2}}O\]
B) \[S{{O}_{2}}\]
C) \[{{H}_{2}}S\]
D) \[{{H}_{2}}Se\]
Correct Answer: A
Solution :
\[{{H}_{2}}O\] has the largest bond angle among VIA group hydrides. The reason is the high electronegativity of oxygen atom due to which bonding electron pair remains closer to oxygen atom in \[{{H}_{2}}O\] molecule. Hence, the repulsion between bonding \[{{e}^{-}}\] is maximum in \[{{H}_{2}}O\] due to which the bond-angle is maximum (104.5°) in \[{{H}_{2}}O\].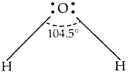
You need to login to perform this action.
You will be redirected in
3 sec
