A) \[s{{p}^{3}}\]
B) \[s{{p}^{2}}\]
C) \[sp\]
D) none of these
Correct Answer: A
Solution :
S atom, in \[SO_{4}^{2-},\] remains \[s{{p}^{3}}-\]hybridized.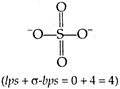 Hence, hybridisation\[=s{{p}^{3}}\]
Hence, hybridisation\[=s{{p}^{3}}\]
You need to login to perform this action.
You will be redirected in
3 sec
