A)
![]()
B)
![]()
C)
![]()
D)
![]()
Correct Answer: C
Solution :
\[-OH\] group in phenol can release electrons to the ring better then\[-C{{H}_{3}}\]group in toluene. \[Cl\] atom has electron withdrawing effect which inhibits electrophilic attack.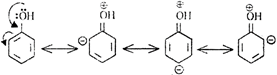
You need to login to perform this action.
You will be redirected in
3 sec
