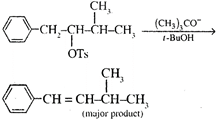
 The correct explanation is:
The correct explanation is:
A) The product is formed due to nucleophilic substitution
B) The product is formed according to Saytzeffs rule
C) Conjugated double bond product is formed due to higher stability because of resonance stabilization
D) \[{{(C{{H}_{3}})}_{3}}C{{O}^{-}}\]is a better leaving group
Correct Answer: C
Solution :
\[{{(C{{H}_{3}})}_{3}}C{{O}^{-}}\]is a better base than a nucleophile. Hence elimination occurs. The product formed is resonance stabilised.You need to login to perform this action.
You will be redirected in
3 sec
