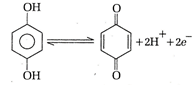
 \[{{E}_{quinhydrone}}\] will be
\[{{E}_{quinhydrone}}\] will be
A) 1.36 V
B) 1.30 V
C) 1.42 V
D) 1.20 V
Correct Answer: C
Solution :
Again, from \[E={{E}^{\circ }}-\frac{0.059}{2}\log {{[{{H}^{+}}]}^{2}}\] \[=1.30-\frac{0.059}{2}\log {{({{10}^{-2}})}^{2}}\] \[=1.30+\frac{0.236}{2}=1.481=1.42\,V\]You need to login to perform this action.
You will be redirected in
3 sec
