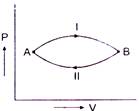
A) \[\Delta {{U}_{1}}>\Delta {{U}_{2}}\]
B) \[\Delta {{U}_{1}}<\Delta {{U}_{2}}\]
C) \[\Delta {{U}_{1}}=\Delta {{U}_{2}}\]
D) \[\Delta {{U}_{1}}=\Delta {{U}_{2}}=0\]
Correct Answer: C
Solution :
Change in internal energy of the system does not depend on path followed. So, \[\Delta {{U}_{1}}=\Delta {{U}_{2}}\]You need to login to perform this action.
You will be redirected in
3 sec
