A) \[KCl{{O}_{4}}\]
B) \[N{{H}_{4}}Cl\]
C) \[C{{H}_{3}}COONa\]
D) None of these
Correct Answer: A
Solution :
Only salts of (weak acid + strong base) and (strong acid + weak base) get hydrolysed (i.e., show alkalinity or acidity in water).\[KCl{{O}_{4}}\]is a salt of strong acid and strong base, therefore it does not get hydrolysed in water. \[KCl{{O}_{4}}{{K}^{+}}+ClO_{4}^{-}\]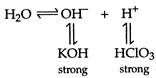
You need to login to perform this action.
You will be redirected in
3 sec
