A) \[N{{H}_{3}}\]
B) \[P{{H}_{3}}\]
C) \[B{{F}_{3}}\]
D) \[PC{{l}_{3}}\]
Correct Answer: B
Solution :
\[B{{F}_{3}}\]is a electron deficient compound. So it has no lone pair orbital over B atom.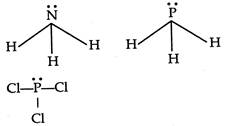
You need to login to perform this action.
You will be redirected in
3 sec
