A) 1
B) \[\frac{1}{2}\]
C) \[\frac{1}{3}\]
D) \[3\]
Correct Answer: C
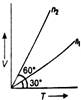
Solution :
Pressure of gas, \[P=\frac{nRT}{V}\] Or \[\frac{V}{T}=\frac{nR}{P}\]ie, slope of graph \[=\frac{nR}{P}.\] Both the gases have same pressure, so, \[n\propto slope\text{ }of\text{ }graph\] \[\therefore \] \[\frac{{{n}_{1}}}{{{n}_{2}}}=\frac{\tan \,{{30}^{o}}}{\tan {{60}^{o}}}=\frac{1}{3}\]You need to login to perform this action.
You will be redirected in
3 sec
