A) a, b, c
B) a, b, d
C) a, c, d
D) b, c, d
Correct Answer: A
Solution :
\[C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}\xrightarrow{NaN{{O}_{2}}+HCl}C{{H}_{3}}c{{H}_{2}}OH\] \[+{{N}_{2}}+NaCl+{{H}_{2}}O\] \[N{{H}_{2}}CON{{H}_{2}}\xrightarrow{NaN{{O}_{2}}+HCl}\]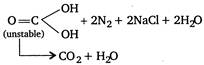 \[C{{H}_{3}}CON{{H}_{2}}\xrightarrow{NaN{{O}_{2}}+HCl}C{{H}_{3}}COOH+{{N}_{2}}\] \[+{{H}_{2}}O+NaCl\]
\[C{{H}_{3}}CON{{H}_{2}}\xrightarrow{NaN{{O}_{2}}+HCl}C{{H}_{3}}COOH+{{N}_{2}}\] \[+{{H}_{2}}O+NaCl\]
You need to login to perform this action.
You will be redirected in
3 sec
