A)
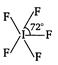
B)
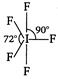
C)

D) None of these
Correct Answer: D
Solution :
Number of hybrid orbitals = number of\[bp\]+ number of\[Ip\] \[=5+1=6\] Thus, hybridisation is \[s{{p}^{3}}{{d}^{2}}\] but geometry, due to the presence of one lone pair, is square pyramidal, ie,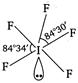
You need to login to perform this action.
You will be redirected in
3 sec
