A) \[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}COO{{C}_{2}}{{H}_{5}}\]
B) \[{{C}_{6}}{{H}_{5}}COO{{C}_{2}}{{H}_{5}}\]
C) \[{{C}_{6}}{{H}_{5}}C{{H}_{2}}COO{{C}_{2}}{{H}_{5}}\]
D) \[{{C}_{6}}{{H}_{11}}C{{H}_{2}}COO{{C}_{2}}{{H}_{5}}\]
Correct Answer: B
Solution :
[b] It is due to presence of \[\alpha \]-hydrogen atom in ester [a]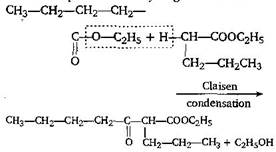 [b] \[{{C}_{6}}{{H}_{5}}COO{{C}_{2}}{{H}_{5}}+{{C}_{6}}{{H}_{5}}COO{{C}_{2}}{{H}_{5}}\] \[\to \] No reaction because for Claisen condensation an ester with two \[\alpha \]-hydrogens atoms are required [c]
[b] \[{{C}_{6}}{{H}_{5}}COO{{C}_{2}}{{H}_{5}}+{{C}_{6}}{{H}_{5}}COO{{C}_{2}}{{H}_{5}}\] \[\to \] No reaction because for Claisen condensation an ester with two \[\alpha \]-hydrogens atoms are required [c] 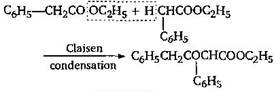 [d]
[d] 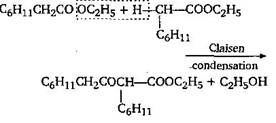
You need to login to perform this action.
You will be redirected in
3 sec
