A) three
B) two
C) six
D) four
Correct Answer: A
Solution :
| In octahedral structure \[M{{X}_{6}}^{,}\] the six hybrid orbitals \[(s{{p}^{3}}{{d}^{2}})\] are directed towards the corners of a regular octahedral with an angle of \[90{}^\circ \]. According to following structure of \[M{{X}_{6}},\] the number of \[XMX\] bonds at \[180{}^\circ \] must be three. |
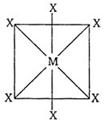 |
You need to login to perform this action.
You will be redirected in
3 sec
