A) \[{{B}_{2}}{{H}_{6}}\]
B) \[{{C}_{2}}{{H}_{6}}\]
C) \[P{{H}_{3}}\]
D) \[Si{{H}_{4}}\]
Correct Answer: A
Solution :
| \[{{B}_{2}}{{H}_{6}}\] is electron deficient molecule because boron atom has three half-filled orbitals in excited state. The structure of \[{{B}_{2}}{{H}_{6}}\] is represented as follows: |
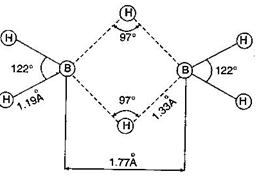 |
| In it two electrons of a \[B-H\] bond are involved in formation of three centre bond, these bonds are represented as dotted lines. |
You need to login to perform this action.
You will be redirected in
3 sec
