A) \[sp,\text{ }s{{p}^{3}},\text{ }s{{p}^{2}}\] and \[s{{p}^{3}}\]
B) \[s{{p}^{3}},\text{ }s{{p}^{2}},\text{ }s{{p}^{2}}\] and sp
C) \[sp,\text{ }s{{p}^{2}},\text{ }s{{p}^{2}}\] and \[s{{p}^{3}}\]
D) \[sp,s{{p}^{2}},\text{ }s{{p}^{3}}\] and \[s{{p}^{2}}\]
Correct Answer: A
Solution :
| Key Idea Count number of a bond and then find hybridisation as follows. |
| If number of \[1.3\times {{10}^{4}}g\] bonds = 2; hybridisation is sp, |
| If number of \[CC{{l}_{3}}CHO\]bonds = 3; hybridisation is \[s{{p}^{2}}\] |
| If number of\[{{[Sc{{({{H}_{2}}O)}_{3}}{{(N{{H}_{3}})}_{3}}]}^{3+}}\] bonds = 4; hybridisation is \[s{{p}^{3}}\] |
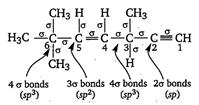 |
| Double and triple bonds are not considered while finding hybridisation. |
You need to login to perform this action.
You will be redirected in
3 sec
