A) \[1.8\times {{10}^{-3}}\]
B) \[3.6\times {{10}^{-3}}\]
C) \[6.0\times {{10}^{-2}}\]
D) \[1.3\times {{10}^{-5}}\]
Correct Answer: C
Solution :
| |
| Equilibrium constant for this reaction, |
| |
| |
| Equilibrium constant for this reaction, |
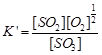 (ii)
(ii) |
| On squaring Eq. (ii) both sides, we have |
| |
| |
| |
You need to login to perform this action.
You will be redirected in
3 sec
